
PLPC™ Platform
Redefining Personalized
Immuno-Oncology:
From Destruction to Reprogramming
A globally audited, patent-protectable immunobiological ecosystem transforming cancer treatment into a scalable, regulation-ready A+ asset class.
immunobiological ecosystem
transforming cancer treatment into a
scalable, regulation-ready
A+ asset class.
engagements with Veristat, Freyr, Freshfields, WilmerHale, Al Tamimi,
and MNS Consulting, forming an institutional network that
ensures regulatory coherence, IP defensibility, and sovereign-level
deployment feasibility.
NOTE: PLPC™️ maintains advanced alignment and collaborative engagements with Veristat, Freyr, Freshfields, WilmerHale, Al Tamimi, and MNS Consulting, forming an institutional network that ensures regulatory coherence, IP defensibility, and sovereign-level deployment feasibility.

Visual Evidence
& Institutional Overview
The PLPC™️ ecosystem has been validated not only through peer-reviewed documentation and independent audits, but also through visual evidence demonstrating its scientific maturity, operational reproducibility, and regulatory readiness.
The following videos form part of the institutional evidence package presented at major international oncology congresses — including ASCO, ESMO, SITC, and CAP-25 — as well as at high-investment biotechnology events such as BioJapan, Dubai 2025, and Abu Dhabi 2025. Their content is based on materials presented during independent third-party audits (Veristat and Freyr) and has been used in strategic investor briefings to illustrate the scientific validation, traceability architecture, and clinical scalability of the PLPC™️ platform and the OncoVix™️ program.
Together, these videos provide a transparent and verifiable overview of how PLPC™️ evolved from a scientific discovery into a transaction-ready, A-class asset — fully validated, globally audited, and prepared for institutional acquisition or licensing.
Video Portfolio — Scientific & Institutional Evidence
Download Executive Summary – PLPC Transaction Overview (PDF)
The Executive Summary condenses the key findings of the 11-year validation process, including:
After reviewing the summary, institutional stakeholders can request complete access to the full set of regulatory, scientific, and financial documentation by contacting:
ops@ogrdalliance.org
Transition to Scientific Evidence
The videos above illustrate the verified structural, regulatory, and institutional maturity of the PLPC™ ecosystem.
The following sections present the documented scientific, regulatory, and economic evidence that support its classification as a fully auditable, non-pharmacodynamic, and acquisition-ready immunobiologic platform — bridging precision oncology, regulatory innovation, and ethical scalability.
The Diagnosis:
When Medicine Forgot to Teach
Every year, more than ten million people are diagnosed with cancer. Half will die not from the disease, but from the toxicity of the treatment.
Chemotherapy destroys tumors — and patients. Radiotherapy burns cancer — and healthy tissue. Surgery removes the mass — but not the memory. Immunotherapy was meant to teach, but it learned to inflame. The global oncology system became a paradox: powerful, expensive, inaccessible. Medicine learned how to destroy, but not how to teach. The human body forgot how to recognize cancer as a threat.
PLPC™ was born from that failure — from the conviction that the immune system does not need to be forced; it needs to be reminded. That the body can learn again.
Más efectiva
Más segura
Más personalizada
Más tratamiento en menos tiempo
Scientific Frustration: The Unfinished Promise
In 2011, the Nobel Prize honored dendritic cells — nature’s teachers of immunity. For the first time, science dreamed of training the immune system to defend itself. The first attempt, Provenge, was brilliant in theory but impossible in practice. It required blood extraction, cell isolation, reinfusion, and cost over two hundred thousand dollars per patient. It was fragile, unscalable, and dependent on living cell cultures. The dream collapsed within five years.
Science had the key, but not the door. PLPC™ built that door.
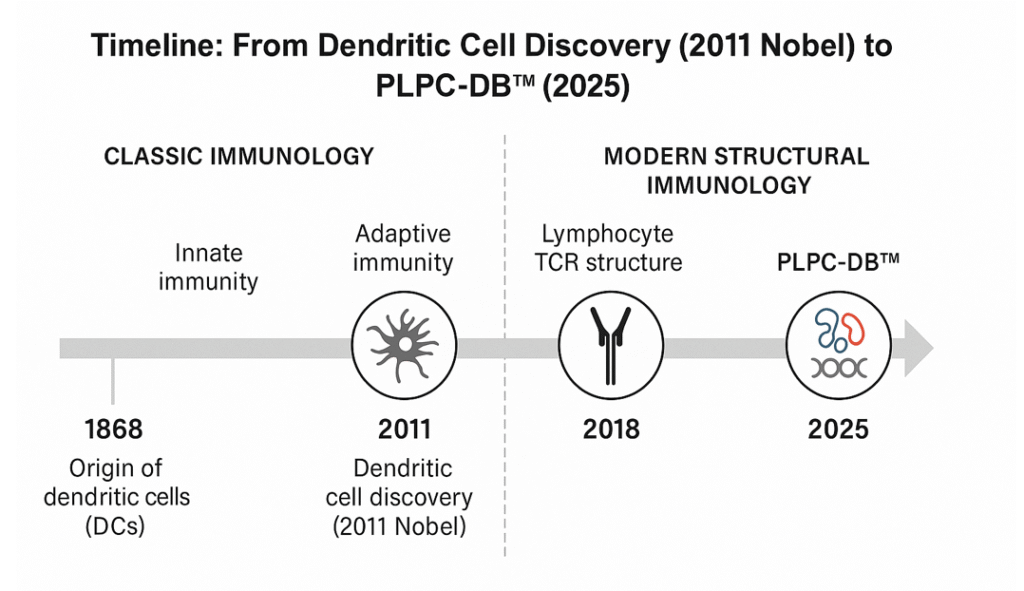
The Solution: PLPC-DB™
Eleven years ago, a multidisciplinary team decided to rewrite that story.
The goal was precise: to create a dendritic-based immunotherapy that needs no cells, no blood, no hospitalization.
We replaced procedures with knowledge.
We used banked PBMCs as a universal source.
We eliminated biopsies through tumor-neoantigen mapping.
We built a stable lyophilized formulation — no cold chain, no fragility.
We designed a dermal delivery system that works in minutes, not weeks.
We removed the hospital from the equation.
The result is PLPC-DB™ — the world’s first non-cellular, antigen-presenting immunotherapy.
It acts from within the body.
It teaches the immune system to recognize and respond.
It activates innate immunity and trains adaptive memory.
That memory detects and blocks residual tumor cells, sustaining durable remission.
This is not theoretical.
It is functional, traceable, and continuously validated under regulatory observation.
Immunotherapy has ceased to be experimental — it is now structural.
Evidence and Validation
From day one, validation was the foundation of PLPC-DB™.
Over 3,500 oncology patients have been observed and audited under real-world evidence design.
Every case was tracked, verified, and analyzed.
Each immune trajectory is measurable and reproducible.
Data from these studies have been presented at ASCO, ESMO, and SITC, and published in five Q1 journals indexed in PubMed.
Three patent families — in the United States, Japan, and Australia — protect its molecular architecture and traceability.
Two pre-FDA audits have confirmed its readiness for submission.
After eleven years of continuous production, PLPC-DB™ is no longer a project; it is a validated bioplatform.

The Scientific Architecture: STIP™
Every innovation requires a system strong enough to prove it.
That system is STIP™ — the Structured Traceability and Immunophenotypic Platform.
STIP™ validates PLPC-DB™ without the need for conventional clinical trials.
It uses human ex vivo models to measure immune activation, cytokine balance, and metabolic recovery.
It complies with 21 CFR Part 11, and integrates New Approach Methodologies (NAMs) recognized by the FDA, EMA, and OECD.
STIP™ compresses a decade of validation into a single documented year.
It reduces the cost of translation by more than fifteen-fold.
With over two hundred modular micro-protocols, STIP™ transforms data into auditable, regulator-ready evidence.
Through STIP™, OGRD Alliance built a new regulatory language: traceable science.
6. The Global Ecosystem: OGRD Alliance
Innovation without structure is fragile.
To sustain PLPC™, we built OGRD Alliance, an international consortium integrating scientific, regulatory, and financial disciplines.
- Latin America manages clinical and real-world evidence.
- The United States leads regulatory engineering and FDA integration.
- Europe coordinates molecular design and quality control.
- Australia safeguards intellectual property.
- Dubai drives strategic expansion and institutional partnerships.
- Panama and Chile anchor production and documentary governance.
OGRD Alliance transforms science into auditable, licensable, and ethically deployable assets.
The Architecture of Structured Immunology
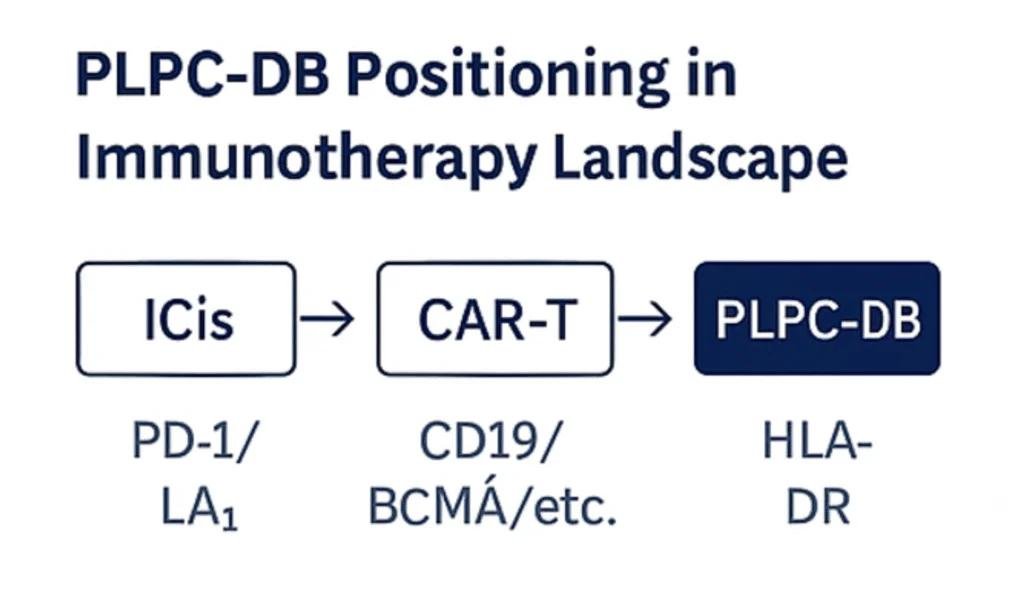
7. Precision Oncology and Personalized Immunotherapy
PLPC-DB™ belongs to a new class of precision immuno-oncology.
It does not attack the tumor with toxicity; it teaches each immune network how to recognize and remember malignant signatures.
It works through immune education, not suppression.
Within the tumor micro-environment, PLPC-DB™ restores communication between dendritic cells, T-cells, and regulatory subsets.
It awakens innate immunity, guides adaptive responses, and establishes long-term immune memory capable of preventing recurrence.
This approach bridges the distance between personalized medicine and standardized manufacturing.
One formulation can adapt immunologically to each patient’s biology — a scalable form of precision immunotherapy.
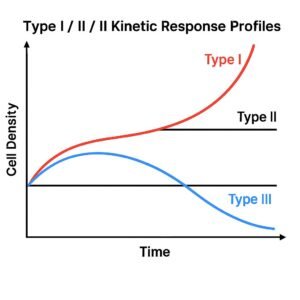
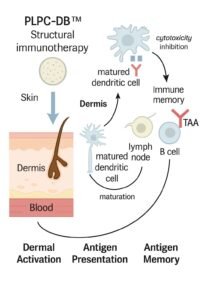
8. How PLPC-DB™ Works
The formulation is a lyophilized matrix of immunomodulatory proteins and phospholipids derived from dendritic-cell secretomes.
It contains no DNA, RNA, or viral vectors — only structure.
Applied intradermally, it engages the most immunologically active interface of the human body: the skin.
Once delivered, its architecture triggers a cascade of immunophenotypic changes:
restoration of antigen presentation, activation of helper and cytotoxic T-cells, regulation of inhibitory cytokines, and establishment of stable immune memory.
The result is a sustained immunological correction rather than a temporary pharmacological effect.
PLPC-DB™ is not absorbed systemically, not metabolized, and not toxic.
It functions locally, through topological signaling, producing measurable activation without inflammation.
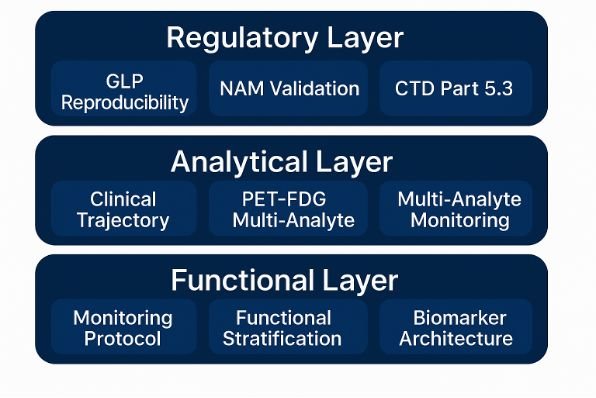
9. STIP™ — The Regulatory Engine
Behind PLPC-DB™ operates STIP™, the Structured Traceability & Immunophenotypic Platform.
It is both scientific and regulatory infrastructure — a platform that allows evidence to exist without the risks of early-phase human trials.
STIP™ integrates:
Functional ex vivo assays measuring immune activation in human cells.
Real-World Evidence (RWE) collected under physician supervision and digital audit.
SAP-secured data architecture compliant with 21 CFR Part 11 and CTD 5.3.
Cross-validation through biomarker reproducibility and imaging correlation.
With STIP™, one year of documented reproducibility replaces ten years of animal and early-human testing.
It has been peer-reviewed in Biomedicines, Biology, Cancers, and IJMS, and audited by Veristat (USA) and Freyr Solutions (Global).
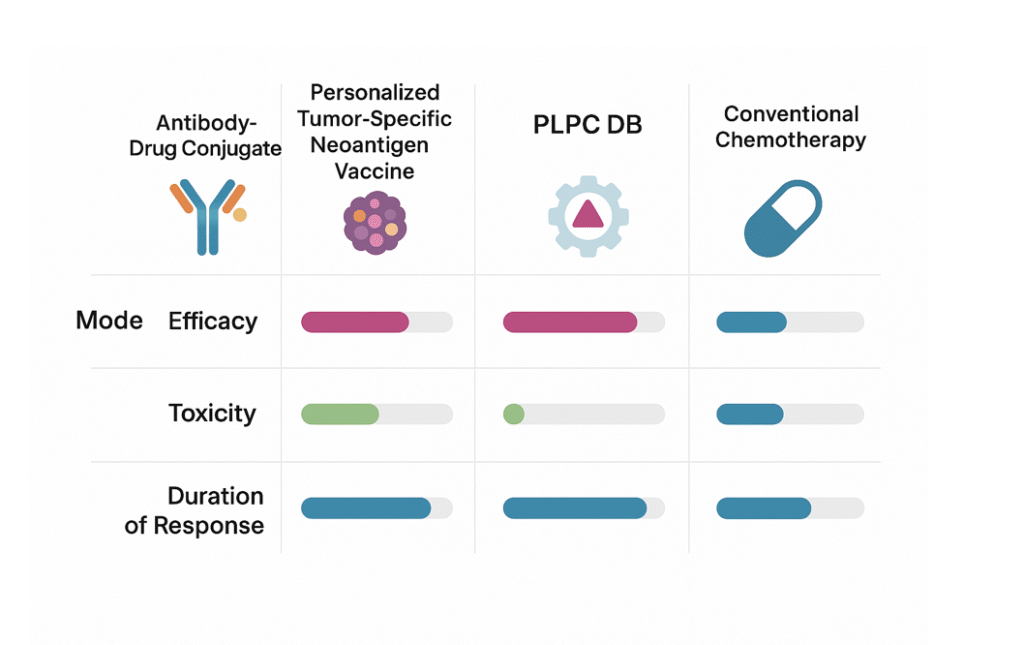
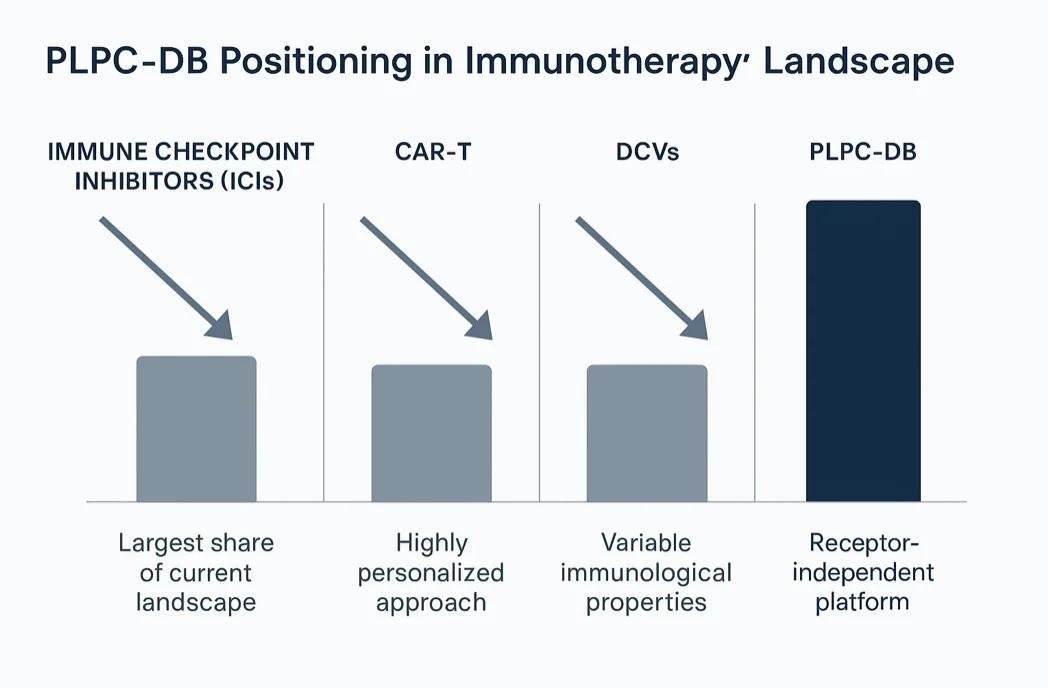
10. The Platform Ecosystem
The PLPC™ ecosystem unites three interoperable pillars:
11. Evidence, Reproducibility, and Safety
The combined data from ex vivo models and audited real-world cohorts demonstrate:
- Consistent activation of immune biomarkers related to tumor recognition and control.
- Sustained functional recovery of exhausted immune profiles in advanced disease.
- Improved metabolic patterns confirmed by imaging follow-up.
- Reproducible results across independent production batches and centers.
- No observed systemic toxicity in thousands of documented administrations.
These outcomes have been independently verified, published, and submitted within pre-IND regulatory dossiers.

12. Global Framework and Intellectual Property
OGRD Alliance coordinates the PLPC™ ecosystem through a comprehensive global structure that unites science, regulation, and finance across six operational regions:
Three patent families — filed in the United States, Japan, and Australia — secure the molecular architecture, structural immunology principles, and documentary framework of PLPC™.
In parallel, proprietary algorithms embedded within STIP™ safeguard its analytical methodology and classification logic, ensuring that the platform remains non-replicable, audit-ready, and regulatorily compliant.
The result is a closed, fully auditable ecosystem connecting laboratory, clinical observation, and regulatory documentation under one continuous and reproducible system.
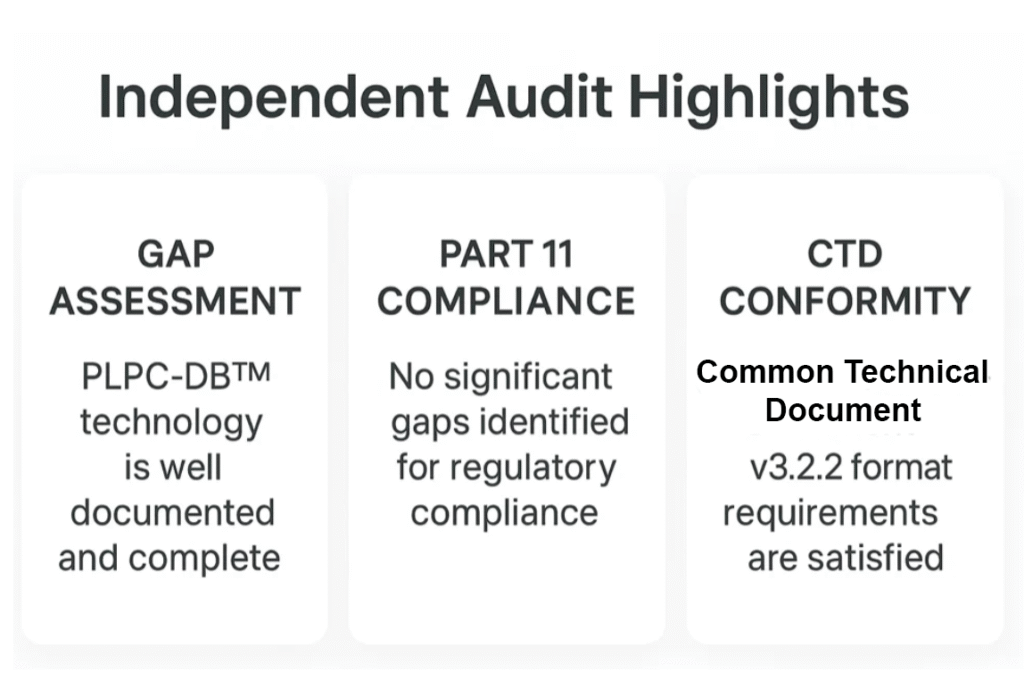
13. Global Validation and Regulatory Readiness
The PLPC™ ecosystem has completed a full scientific and regulatory validation cycle, meeting every standard required for institutional readiness.
- Scientific validation: Five Q1 peer-reviewed publications — Cancers, Biomedicines, Biology, IJMS, and MethodsX — document the immune kinetics, structural logic, and reproducibility of the platform.
- Regulatory integration: Fully formatted CTD Module 5.3 aligned with FDA 505(b)(1), EMA Early-Access, and OECD NAM standards, representing the first ex vivo–based immunobiologic submission model of its kind.
- Audit confirmation: Independent gap analyses by Veristat (USA) and Freyr (Global) confirmed compliance with 21 CFR Part 11, ICH Q6B, and CTD v3.2.2, certifying PLPC™ and STIP™ as “immediately bankable / audit-ready.”
- Intellectual protection: Three active patent families covering composition, function, and documentation methodology.
Through these milestones, PLPC-DB™ achieved what most bioplatforms require decades to reach: a validated, non-toxic, non-genomic, fully documented immunotherapy platform — a new class of structural biologic recognized under the FDA Modernization Act 2.0.
14. Public Documentation and Open Due Diligence
The PLPC™ platform is supported by an extraordinary level of scientific transparency.
Its entire development history, from early ex vivo validation to regulatory readiness, is already published, audited, and publicly accessible — forming an open-source due diligence archive unprecedented in this industry.
All foundational data are contained in peer-reviewed journals, indexed in PubMed, and freely available for institutional verification.
These publications collectively demonstrate not only functional reproducibility, but also the maturity of PLPC™ as a fully evidenced and regulatorily aligned immunobiologic.
Commitment to Innovation and Progress
Each of these documents is open access, citable, and indexed, providing verifiable proof of reproducibility and scientific integrity.
Together, they form an independent, public validation layer that replaces speculative due diligence with open, peer-reviewed evidence.
No comparable biotechnology asset of similar valuation offers a transparency framework of this depth or accessibility.
15. Impact in Precision Oncology
PLPC-DB™ introduces structural immunology into the field of precision oncology.
Unlike pharmacological or cell-based therapies, it activates immune recognition through architecture, not aggression.
It reprograms the tumor microenvironment, restores immune communication, and establishes durable Th1 memory — measurable through non-destructive biomarkers and PET-FDG imaging.
This is personalized immunotherapy without genetic manipulation — precision achieved through structural intelligence.
Its safety profile, accessibility, and reproducibility allow application across patients traditionally excluded from clinical trials: elderly, fragile, or heavily pretreated individuals.
The platform converts what used to be exceptional cases into measurable, standardized responses, positioning PLPC™ as a cornerstone in precision immuno-oncology.
16. Scalability and Ethical Transformation
PLPC-DB™ is stable at room temperature, manufactured under GMP, and validated through STIP™, eliminating the logistical barriers that limit conventional biologics.
Each batch is identical in fingerprint and performance, documented through inter-batch functional validation and SAP-integrated version control.
Ethically, the system eliminates the two major sources of risk in biomedical innovation:
- No animal testing — validated exclusively through human ex vivo systems.
- No early-phase human exposure — governed entirely by RWE and NAM frameworks.
This is not invasive medicine; it is structurally continuous medicine — reproducible, ethical, and future-proof.
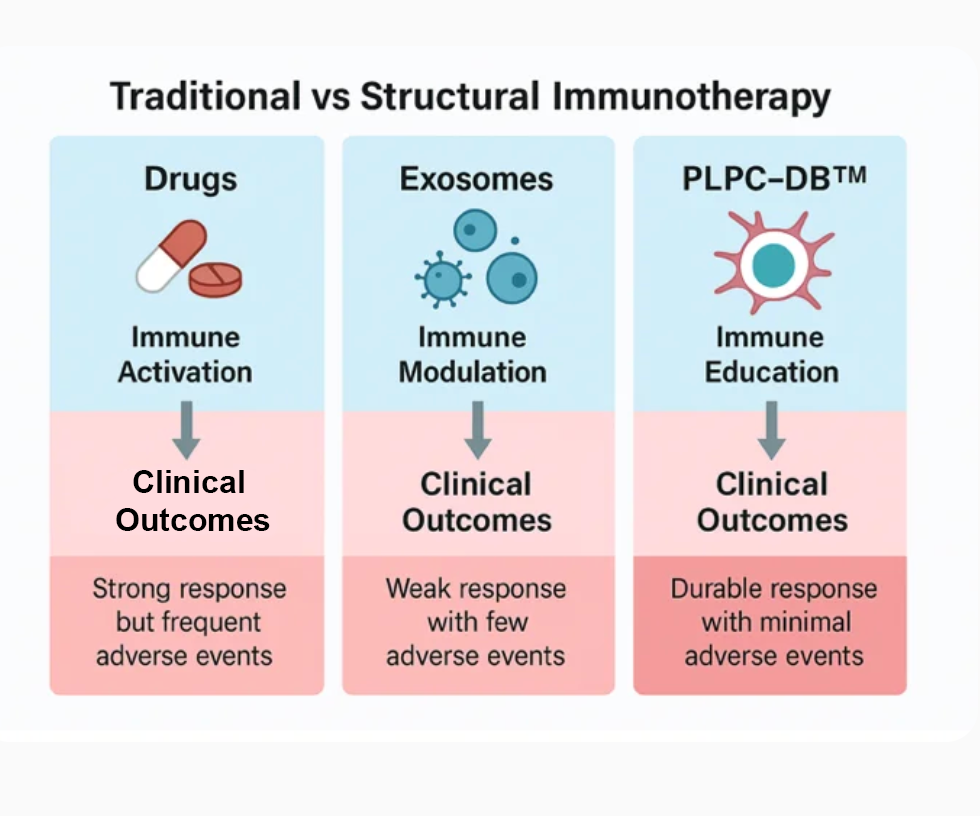
17. Consolidated Valuation and Institutional Readiness
Independent analyses (eBrief 0 – PLPC Platform; Corporate Declaration Act 2025) classify the PLPC™ ecosystem as a Composite A-Class Asset:
18. Vision: The Era of Structured Immunity
PLPC™ and STIP™ define the transition from destructive medicine to educational medicine. They bring precision to immunotherapy, structure to biology, and ethics to innovation.
This is oncology without toxicity, immunotherapy without inflammation, and science without opacity. The body was never designed to be at war with itself — it simply needed to remember the language of defense. That language now has structure.
PLPC™ – The Future of Immunity Is Now Measurable.
Transaction Readiness & Public Due Diligence Archive
The PLPC™ ecosystem is not an exploratory biotechnology project — it is a Class A+ institutional asset, fully validated, peer-reviewed, audited, and ready for transaction or acquisition.
All technical, regulatory, and corporate evidence has been pre-compiled into a public due diligence archive, accessible for institutional verification.
This open-data structure — unprecedented in the sector — allows investors, regulators, and acquirers to review the entire audit and valuation chain prior to engagement, confirming that PLPC™ is transaction-ready, audit-certified, and regulatorily aligned under FDA & NAM frameworks.
Requests for access to restricted files or data-room credentials must be addressed to ops@ogrdalliance.org
(subject: “Request – PLPC Documentation Set [Entity / Country]”).
Certain files may require a confidentiality agreement (NDA).
Transaction Readiness & Public Due Diligence Archive
Public Due Diligence Index – Available Files
The PLPC™ ecosystem is a Class A+ institutional asset, fully validated, peer-reviewed, and transaction-ready.
All technical, scientific, regulatory, and financial components are pre-audited and structured into a public due diligence archive for acquisition, licensing, or institutional investment.
Requests and restricted-access credentials: ops@ogrdalliance.org
(subject: “Request – PLPC Documentation Set [Entity / Country]”)
Certain documents may require NDA execution prior to delivery.
Download here a brief synopsis of the Data Room contents.
To request full access, please contact us by email.
Download here
Summary of Status
Scientific Publications, International Congresses & Books
The PLPC™ ecosystem is supported by a robust public scientific record, with presentations and peer-reviewed publications across Tier-1 international congresses, Q1 journals, and Amazon-published scientific works.
This record consolidates the platform’s global recognition, transparency, and maturity as a Class A+ transaction-ready asset.
A. Summary of Institutional Validation and Asset Status
B. Amazon Books and eBooks
C. International Tier-1 Congresses
ASCO — American Society of Clinical Oncology
SITC — Society for Immunotherapy of Cancer (USA)
ESMO — European Society for Medical Oncology (Europe)
CAP25 — College of American Pathologists Annual Meeting (USA)
Summary
This portfolio of books, congress presentations, and scientific publications demonstrates that the PLPC™ ecosystem has moved beyond the exploratory stage.
Its innovation has been recognized, audited, published, and replicated at international scale — a unique position among global immunotherapy assets.
Strategic Instruction Horizon — The True Cost of Comprehension
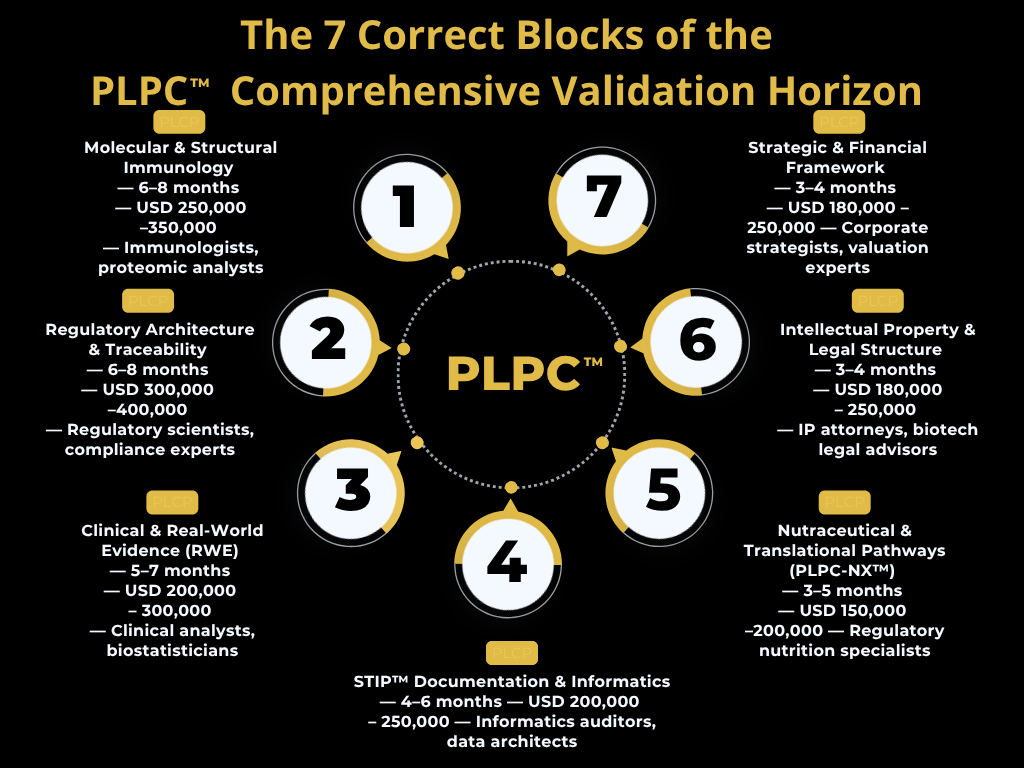
Understanding the PLPC™ ecosystem is not a technical exercise — it is a structured process of intellectual assimilation.
The system integrates immunobiologic science, regulatory architecture, digital traceability, nutraceutical translation, and intellectual property — dimensions that together define its valuation, legitimacy, and institutional scalability.
Reaching a level of comprehension equivalent to what PLPC™ offers through its own Preferential Access Framework would require an extensive, multidisciplinary instructional process — theoretical, non-operational, and focused exclusively on understanding the ecosystem as an investment-grade scientific asset.
Institutional Context
This projection represents the theoretical cost of comprehension — not production, not commercialization, not application.
Even with global access to consulting firms and academic partners, achieving equivalent understanding across all seven domains would require nearly two years and at least two million USD in structured instruction, with no rights to operate, replicate, or monetize the technology.
The PLPC™ Preferential Access Framework, offered at a Preferential Savings Price, condenses that extensive theoretical horizon into a two-day guided immersion — enabling institutional understanding of an A+ audited bioplatform without operational exposure or regulatory risk.
Knowledge Without Risk — Comprehension Without Exposure
What others would need years to decode, PLPC™ delivers in two days of structured, regulatorily aligned access.
It is not production training — it is intellectual orientation: a window into how validated science becomes an investable, acquisition-ready system.
PLPC™ — 11 Years of Research, Two Years of Global Validation, and the Complete Institutional Understanding Delivered in Two Days.
Structured. Safe. Preferentially Accessible.
Email: ops@ogrdalliance.org
Connect with Us
OGRD Alliance
Scientific & Regulatory Coordination Unit
📧 ops@ogrdalliance.org
🌐 www.ogrdalliance.org
🌐 www.plpc-db.com
🌐 www.oncovix.com
🌐 www.abimprosyc.com
🌐www.drramongutierrez.com
- COMPANY: OGRD Alliance L.L.C-FZ; ADDRESS: Meydan Grandstand, 6th Floor, Meydan Road, Nad Al Sheba, Dubai, U.A.E.




